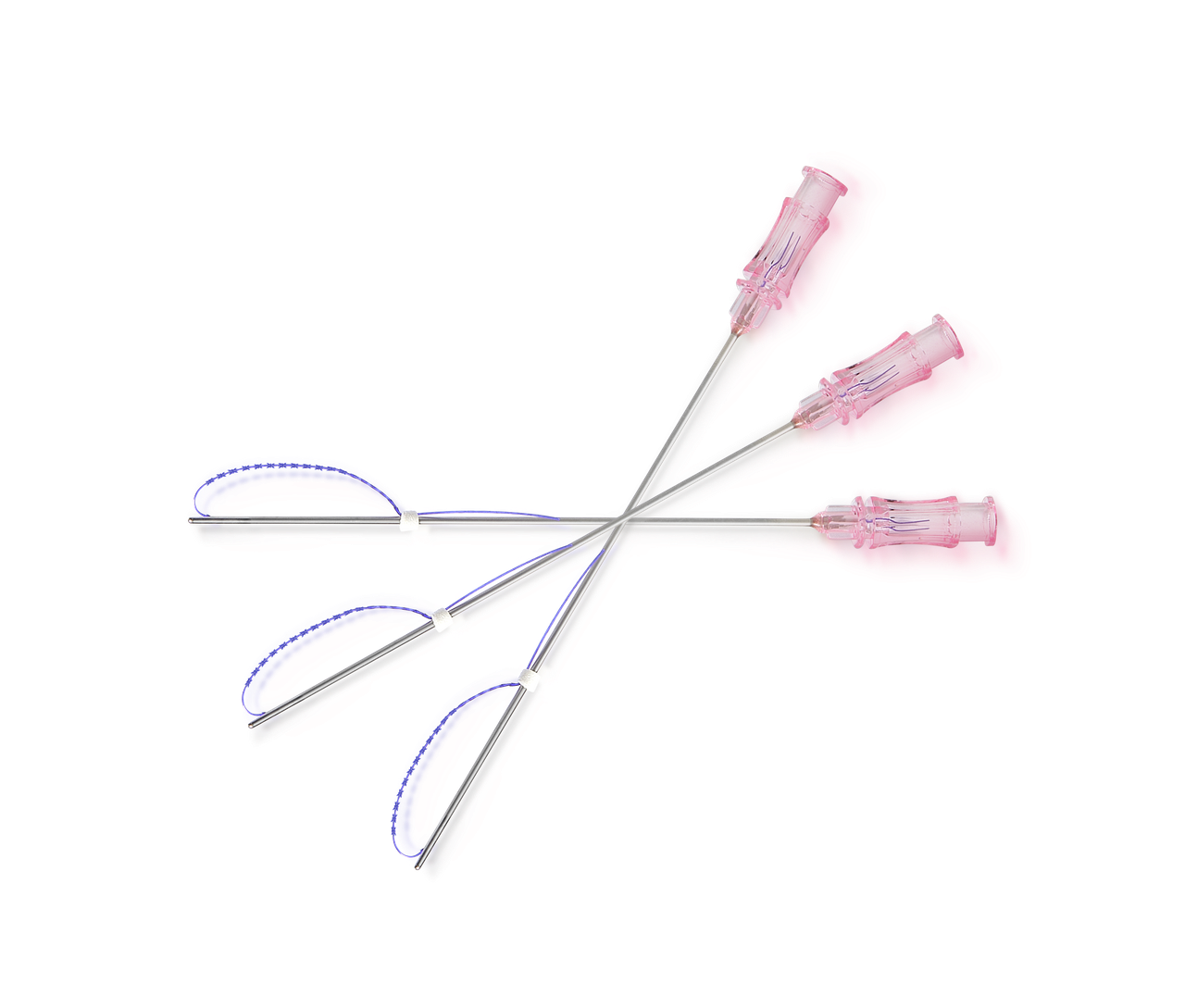PRODUCT INFO
Superior Traction Power and Holding
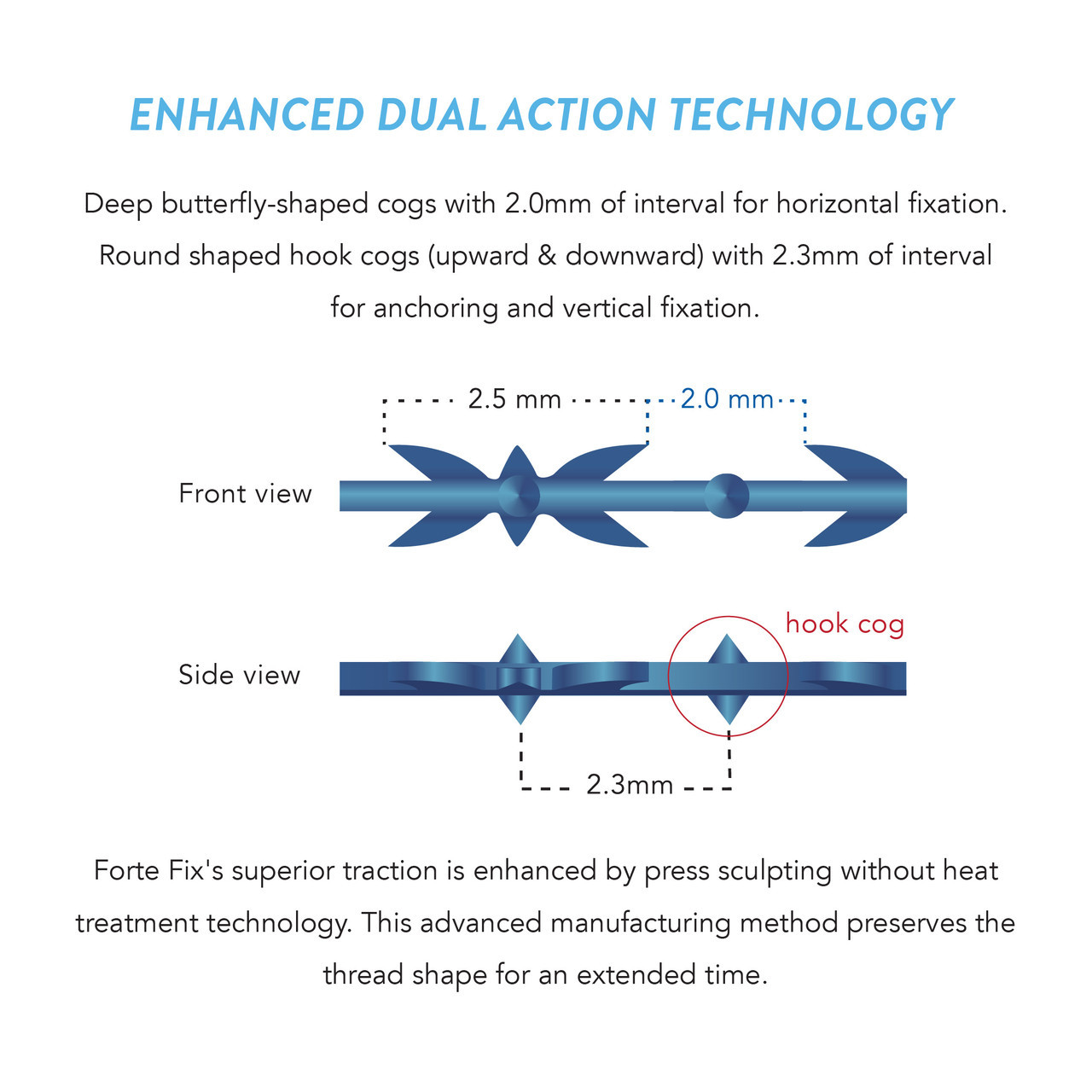
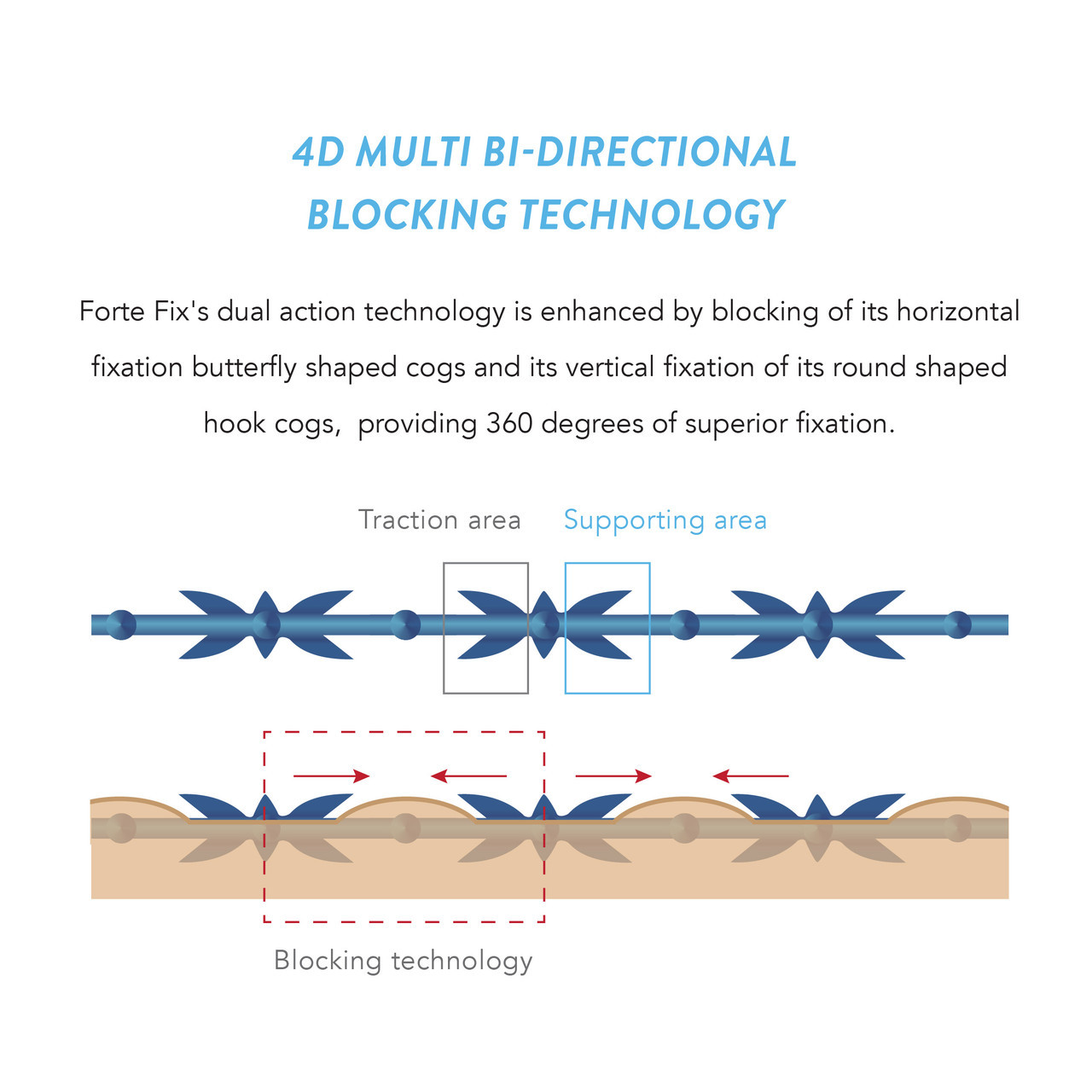
Forte Fix provides superior traction, hold and fixation through 4-Dimensional multi bi-directional blocking technology with heatless press sculpting. This innovative technology raises the hypodermis more evenly by balancing the movement of the central volume through selective targeting of the Traction and Supporting Areas.
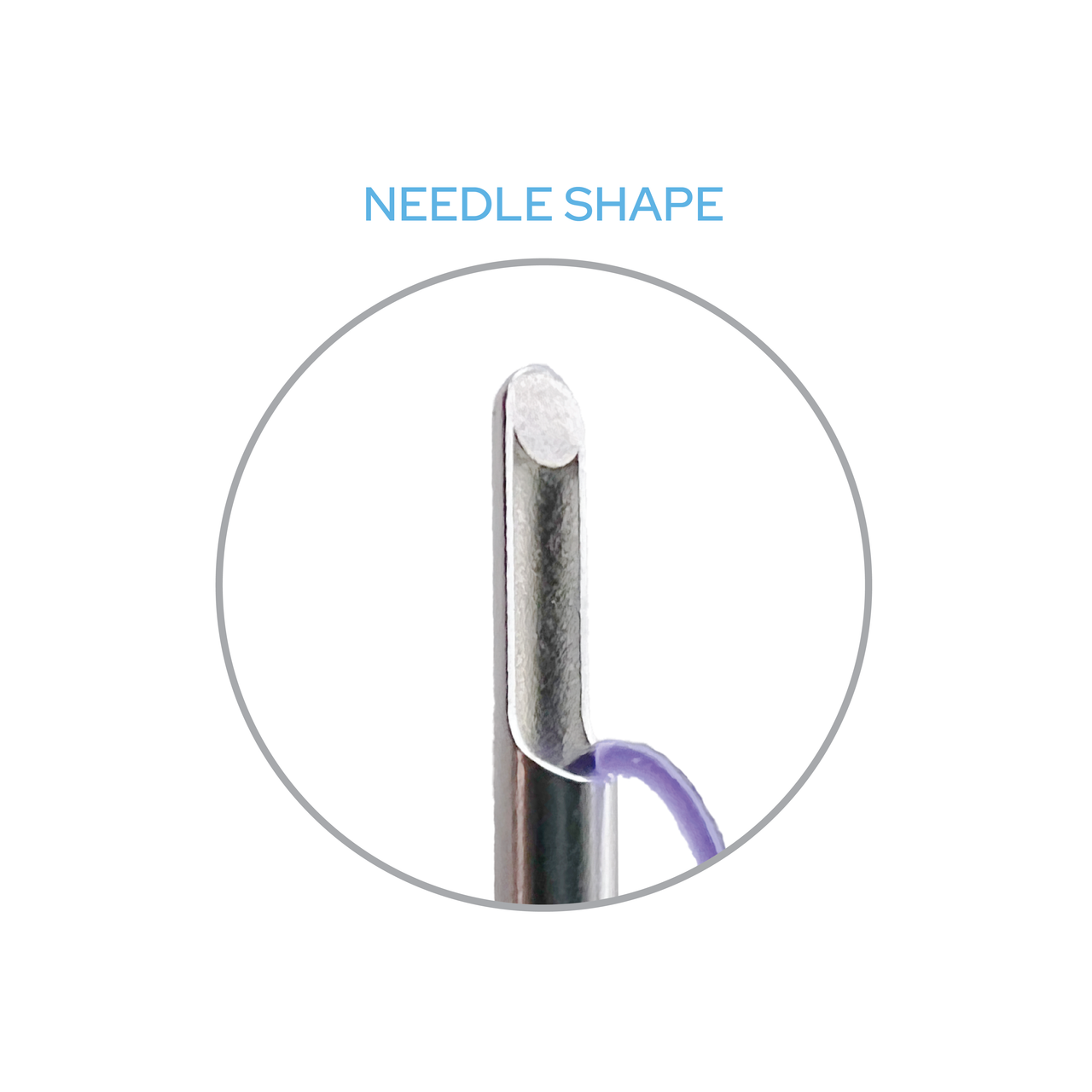
Needle shape: FCL Cannula
Thread (USP x Length): USP 2-0 x 185mm / USP 3-0 x 160mm
Thread specs:
- 19G x 70mm
- 18G x 100mm
INDICATIONS
Injection with PDO is indicated for soft tissue augmentation where the insertion of surgical sutures is appropriate. Injection of PDO threads is indicated for subcutaneous (intradermal and hypodermal) implantation.
PACKAGING & STORAGE
KEEP OUT OF REACH OF CHILDREN. All Threads should be stored in their original, unopened pouches and foil bags, in a temperature-controlled room, away from direct sunlight.
The outer re-sealable foil bag protects sutures from light and humidity. The inner pouches are for sterility. You can store unopened pouches in the re-sealable foil bag. Opened pouches should be used within 24 hours. Opened/resealed foil bags and its contents should not be used after 8 weeks.
- The contents of each package have been sterilized by Ethylene Oxide. Product is non-toxic and non-pyrogenic meaning product is non-flammable
- This product is for single Use Only. Please discard immediately after opening. Do not use if package is open or damaged as sterility may be compromised
- Product is sensitive to moisture so if bag is not properly sealed, product viability will be compromised
- Each package contains a lot number, manufacturing date and product expiration date. Information is located on bottom of the back label.
CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS
MIRACU PDO Threads® should not be used in patients with any known allergy or foreign body sensitivities to polydioxanone or poly-l-actic sutures or in situations where internal fixation is otherwise contraindicated. The device should also, not be used in patients appearing to have very thin or soft tissue in which implant may become visible. Implantation of foreign materials into tissue can result in histological reactions. Do not use in patients that are allergic to MIRACU PDO Threads®.
A review of the patient’s medical history including, but not limited to, medical problems, allergies, history of previous treatments, and procedures at the site of the treatment area should be conducted during the patient’s assessment. Upon review of the assessment, the following protocols related to indications, contraindications and exclusions should be observed (see package labeling for individual product information).
The injection of PDO threads is contraindicated in the following conditions (see manufacturer’s manual for product information and individual treatment recommendations):
- Pregnancy and breast feeding
- The presence of infection or any other inflammatory condition at the proposed treatment site caused by open sores, rashes or breakouts.
- Severe allergies of any kind.
- A history of hypersensitivity or allergic reaction to surgical sutures
- A history of repeated unsuccessful treatments with PDO threads
- A history of anaphylaxis or anaphylactoid reaction to injected products
- A history of non-compliance with post-injection instructions
- Intoxication or influence of illicit drugs
- Not suitable for patients with Hepatitis B and C, HIV.
- Active acne, cystic acne and vulgaris acne.
- A predisposition to Keloid scarring.
- Malignant cancer or any treatments for cancer.
- Diseases such as scleroderma, sarcoidosis, amyloidosis.
- Diseases like diabetes and tuberculosis.
- Patient cannot be on Anti-coagulants, Coumarin, Heparin.
- Excessive preoccupation with a minor defect of face or body.
- Porphyria; a group of rare inherited blood disorders that effect hemoglobin.
- Patients with Hemophilia should not be treated with threads.
- Use with caution in patients with various acute infectious diseases (SARS, influenza, etc.)
- Do not use in patients with a non-absorbable implant (Silicone) in zone of desired treatment area
DISCLAIMER:
Miracu™ barbed surgical sutures are FDA cleared for soft tissue approximation where use of an absorbable suture is appropriate. (K172602)
Additional information
| Weight | 112.6 g |
|---|---|
| Dimensions | 260 × 190 × 20 mm |
| Gauge & Length * | 18G X 100mm, 19G X 70mm |
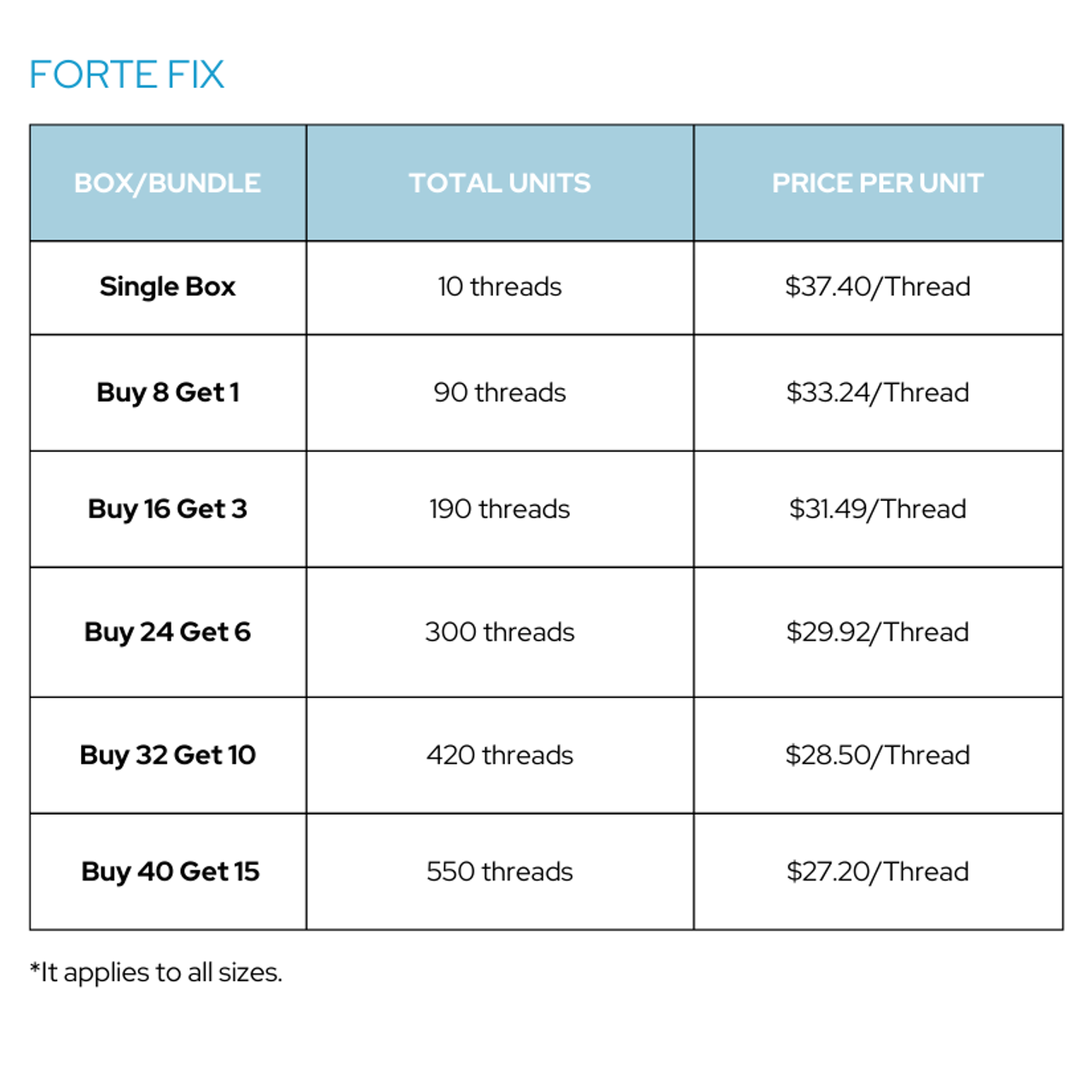
| Box/Bundle * | Single Box |
Miracu
Return & Exchanges
This Product Return Policy (“Product Return Policy”) is for all Products (“Product” or “Products”) distributed in the United States by DBM Corporation Inc the Official US Representative of MIRACU PDO Threads (TM). Note that this Product Return Policy contains distinct terms and conditions for returns from entities that purchase product from DBM Corporation Inc directly or an authorized DBM Corporation Inc sales representative. Our Returns Policy does not authorize return or exchange on an item bought through an authorized representative sales representative or via the DBM Corporation website.
Shipping Policy
Miracu PDO thread orders are shipped Monday – Friday. Orders received after 2PM PST will be shipped on the next business day. *Subject to change